The mtFIT test detected more advanced adenomas than FIT, indicating that mtFIT could be more effective in population based colorectal cancer screening
AMSTERDAM, NETHERLANDS, February 10, 2024 /EINPresswire.com/ — Each year, worldwide 1,8 million people develop colorectal cancer (CRC) and half of them die from it
– Early detection (“screening”) & removal of advanced polyps or early cancer is the most effective approach
– The Fecal Immunochemical Test (FIT), the most widely used screening test, is effective but misses most advanced adenomas
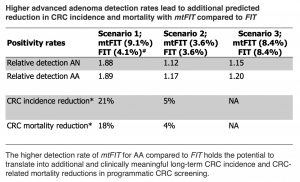
– mtFIT detects 17-20% more advanced adenomas than conventional FIT, which could translate into 5-20% incidence and 4-18% mortality reduction
– Data are published in the prestigious journal The Lancet Oncology
PROSPECTIVE EVALUATION IN THE SETTING OF THE DUTCH NATIONAL CRC SCREENING PROGRAM
CRCbioscreen, a Dutch diagnostics company, dedicated to developing a next generation stool test for population based ColoRectal Cancer or CRC screening, today announced the publication of positive results of a population-based paired-design intervention study with a multitarget immunochemical test (mtFIT) for early detection of CRC. Data were published in the prestigious journal The Lancet Oncology.
The researchers found that detection rates for advanced adenomas, that make up the vast majority of advanced colorectal neoplasia, were at least 17% higher for mtFIT than for FIT in three different scenarios, depending on the positivity rate chosen. Long-term modelling revealed that mtFIT-based screening could be cost-effective at a realistic price level and reduce CRC incidence by 5-20% and CRC-related mortality by 4-18%, compared to FIT-based screening.
The paper was authored by a team of researchers and clinicians from the Netherlands Cancer Institute, Amsterdam, in collaboration with researchers from AmsterdamUMC and ErasmusMC in Rotterdam, The Netherlands.
Individuals eligible for the Dutch national FIT-based CRC screening program, i.e. men and women aged 55 to 75 years, were invited, between March 25th 2022 and December 7th 2022, to submit both a FIT and an mtFIT sample collected from the same bowel movement. Positive FIT (47 μg/g haemoglobin cut-off) and/or mtFIT (based on combining hemoglobin, calprotectin and serpin F2 measurements in a decision tree algorithm) led to colonoscopy referral. This study aimed to validate mtFIT versus FIT.
Primary outcome was the relative detection rate of advanced neoplasia (AN) across multiple test positivity rates. Key secondary outcomes were the relative detection rates of CRC, AA and ASP across multiple test positivity rates. Long-term health and economic impacts were determined using microsimulation modelling. The study has been registered in ClinicalTrials.gov (NCT05314309) and has been completed.
The higher detection rate of mtFIT for advanced adenoma (AA) compared to FIT holds the potential to translate into additional and clinically meaningful long-term CRC incidence and CRC-related mortality reductions in programmatic CRC screening.
Gerrit Meijer, MD, PhD, CRCbioscreen’s CSO and principal investigator, commented:
“We are very pleased with the results of this population-based paired-design intervention study. There is a need for a noninvasive screening test like mtFIT that has higher sensitivity for precursor lesions without increasing false-positive test results. This multitarget protein-based test shows a significant improvement over FIT in detecting advanced adenomas, leading to a predicted important further reduction of CRC incidence and mortality with mtFIT-based screening compared to FIT-based screening. Importantly, the test logistics still remain fully compatible with that of current FIT-based population screening programs. Early detection of colorectal cancer can save many lives. These data underscore our commitment to contribute to a substantial worldwide reduction in incidence and deaths due to this life-threatening disease.”
Meike de Wit, PhD, CRCbioscreen’s COO and corresponding author, adds:
“These results provide a solid basis for further developing the multitarget FIT into a clinical-grade assay, ready to serve as thenext-generation CRC screening test.”
Henri Theunissen, PhD, CRCbioscreen’s CEO: “This study provides evidence for the medical relevance but also the commercial opportunity of mtFIT. We will be working with partners to fund product development and go to market as soon as possible.”
ABOUT CRCbioscreen
CRCbioscreen is a spinoff from the Netherlands Cancer Institute. CEO Dr. Henri Theunissen is a science business expert who has started and grown over 30 spinoff companies. CSO Professor Gerrit Meijer and COO Dr. Meike de Wit have longstanding experience in the field of colorectal cancer detection and biomarker development. The associated research team has published over one hundred papers related to colorectal cancer screening. CRCbioscreen is dedicated to developing mtFIT as a unique, next-generation test that measures three different biomarkers in stool. mtFIT has been shown to be a cost-effective diagnostic test that can add great value to programmatic population-based colorectal cancer screening. Investors in CRCbioscreen include The Mark Foundation for Cancer Research and Human+ (www.humanplus.org).
Henri Theunissen
CRCbioscreen
[email protected]
Visit us on social media:
LinkedIn
![]()
Article originally published on www.einpresswire.com as CRCbioscreen Announces Positive Results of Population-Based mtFIT Study for Early Detection of Colorectal Cancer